Perspectives: Competing to Win Versus Collaborating to Succeed?
By John Wingard, MD
 Several months ago, a patient with myelofibrosis was referred to me to advise when she should be transplanted. I find myelofibrosis to be challenging to decide about transplant. One needs to achieve a Goldilocks type of decision: Not too soon, not too late, just right! Waiting until transformation to acute leukemia is too late. Taking the patient to transplant at a time when she is at low risk is too early. Proceeding to transplant when the patient is at an intermediate risk seems to be the sweet spot, balancing risk and benefit. Multiple studies have shown that several prognostic algorithms can assist in making transplant decisions. I ordered new generation sequencing (NGS), judging the mutation-enhanced international prognostic scoring system (MIPSS) or genetically inspired prognostic scoring system (GIPSS) prognostic algorithms to be the most accurate.
Several months ago, a patient with myelofibrosis was referred to me to advise when she should be transplanted. I find myelofibrosis to be challenging to decide about transplant. One needs to achieve a Goldilocks type of decision: Not too soon, not too late, just right! Waiting until transformation to acute leukemia is too late. Taking the patient to transplant at a time when she is at low risk is too early. Proceeding to transplant when the patient is at an intermediate risk seems to be the sweet spot, balancing risk and benefit. Multiple studies have shown that several prognostic algorithms can assist in making transplant decisions. I ordered new generation sequencing (NGS), judging the mutation-enhanced international prognostic scoring system (MIPSS) or genetically inspired prognostic scoring system (GIPSS) prognostic algorithms to be the most accurate.
The insurance company declined to authorize the test. The letter stated the request was reviewed by a nurse with special training in the applicable rules of the insurance plan and a pediatrician. They decided that NGS testing was experimental for myelodysplasia.
Our patients rely on us to make careful decisions on their behalf. We have a responsibility to do our best to give them a considered recommendation. The patient deserves nothing less. Besides the denial, I was angered that the insurance company had not seemed to exercise the same care in making a considerate decision as I was trying to: An adult disease reviewed by a pediatrician who may not even have any oncology training, much less experience with myelofibrosis? A nurse with no purported experience in oncologic testing? They even had the disease wrong: Myelodysplasia rather than myelofibrosis?
Naturally, I appealed the decision on my patient's behalf and provided two published articles demonstrating the superior prognostic accuracy of algorithms that incorporate testing for high molecular risk mutations (included in the MIPSS70 and GIPSS algorithms). The appeal was denied again. This time I was informed the basis is that there are quality concerns about the performance of NGS; the letter cited a paper nearly 10 years old. The reviewer was not cognizant of advances in NGS testing, much less the clinical laboratory improvement amendments (CLIA) regulations that govern lab testing to ensure quality procedures and controls are in place.
Again, I appealed, now several months into this test of wills. It seemed that the protracted pace of this process was designed to encourage me to move on to something else and abandon the request. I expected an opportunity for a "peer-to-peer" conversation.
But, no, this time "the case" was kicked to a state administrative law hearing before a judge. I was served papers and interrogatories to complete by opposing counsel. Well, this was new to me! But, at least I will have my day in court in late January.
I confess the legal profession perplexes me. It seems to me to be so different than medicine. From my perspective, it seems that legal decisions are made by two sides arguing with each other. The combatants try to win, to defeat the other. Winning the case is the goal! The goal is not what is fair, what is true, what is best; those seem to be hoped-for byproducts that may accrue to the winning side. It seems that this frame of reference is foreign to medical decision-making. At our best, we strive to take into consideration as much input as possible to reach the best decision. At our best, we make decisions collaboratively, in tumor boards, or multidisciplinary conferences. Winning is not in the equation.
So, here on the cusp of the New Year as I write this, I reflect on this unexpected complexity as to how to make the best decision. I find myself dragged into a legal exercise crawling at a glacial pace with an emphasis on combat and winning: I am reduced into hoping, for my patient's sake, I win.
Return to TOP
CIBMTR Aids Approval of First Drug to Prevent Acute GVHD: Leslie Kean, MD, PhD, to Present Poster at 2022 Tandem Meetings
By Jennifer Motl
 Based in part on real-world evidence from the CIBMTR, the US Food and Drug Administration (FDA) recently approved abatacept (Orencia) for the prevention of acute GVHD in patients aged 2 and older who undergo allogeneic HCT from an unrelated donor.
Based in part on real-world evidence from the CIBMTR, the US Food and Drug Administration (FDA) recently approved abatacept (Orencia) for the prevention of acute GVHD in patients aged 2 and older who undergo allogeneic HCT from an unrelated donor.
Dr. Leslie Kean, MD, PhD, of the Dana-Farber Cancer Institute, will present a poster1 about this research in April at the 2022 Tandem Meetings I Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR. Attendees can preview the abstract and submit questions to Dr. Kean by logging into the 2022 Tandem Meetings site and searching for "improved overall survival in patients treated with abatacept." Also, the CIBMTR recently posted a public plain-language summary of this research.
"Real-world data from the CIBMTR was one component of determining clinical effectiveness of abatacept, emphasizing the impact our database has on improving outcomes for patients," said Linda J. Burns, MD, Senior Scientific Director, CIBMTR MCW.
"We used an Inverse Probability Treatment Weighting (IPTW) regression analysis with propensity score weighting to minimize any potential treatment selection bias caused by using real-world control data from CIBMTR," said Mei-Jie Zhang, PhD, Chief Statistical Director, CIBMTR MCW.
Second, only to relapse, severe acute GVHD is a common cause of death after allogeneic HCT. In the past, people needed a closely matched donor to reduce their chances of developing acute GVHD. People of color are more likely to have a mismatched rather than closely matched donor. Using abatacept to prevent acute GVHD may allow more people to get life-saving transplants, even with a mismatched donor.
Abatacept was previously approved for the treatment of rheumatoid arthritis and other autoimmune conditions. Bristol Myers Squibb (BMS), the manufacturer of abatacept, asked for FDA approval to use abatacept to prevent acute GVHD. In turn, the FDA requested real-world evidence of overall survival 180 days post-transplant. BMS then sponsored a corporate study by physicians and statisticians at CIBMTR MCW.
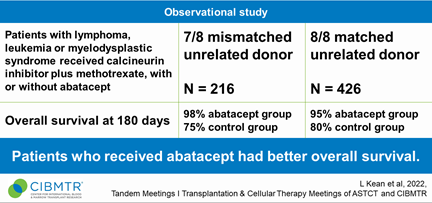
Researchers observed 216 people who had 7/8 HLA-mismatched donors and 426 people who had 8/8 closely matched donors, during 2011-2018. Patients had leukemia, lymphoma, or MDS. They received calcineurin inhibitors plus methotrexate, with or without abatacept.
Patients treated with abatacept had significantly better overall survival at Day 180 compared to controls. Results of this real-world study were similar to the phase 2 study2, the other component of the FDA determination of clinical effectiveness. Results were also presented in part at the ASH Annual Meeting3 in December.
References
- Kean LS, Burns LJ, Kou TD, et al. Improved overall survival of patients treated with abatacept in combination with a calcineurin inhibitor and methotrexate following allogeneic hematopoietic stem cell transplantation: Analysis of the Center for International Blood and Marrow Transplant Research Database. Tandem Meetings | Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR; In press. 2022.
- Watkins B, Qayed M, McCracken C, et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. Journal of Clinical Oncology. 2021 Jun 10; 39(17):1865-1877. Epub 2021 Jan 15. doi: 10.1200/JCO.20.01086.
- Kean LS, Burns LJ, Kou TD, et al. Improved overall survival of patients treated with abatacept in combination with a calcineurin inhibitor and methotrexate following 7/8 HLA-matched unrelated allogeneic hematopoietic stem cell transplantation: Analysis of the Center for International Blood and Marrow Transplant Research Database. Blood. 2021;138(Supplement 1):3912-3912.
Return to TOP
Publicly Available Datasets
In accordance with the NIH Data Sharing Policy and NCI Cancer Moonshot Public Access and Data Sharing Policy, the CIBMTR makes the final datasets from published studies publicly available on the CIBMTR Research Datasets for Secondary Analysis webpage. These publication analysis datasets are freely available to the public for secondary analysis.
The CIBMTR launched the webpage in July 2019, and as of January 25, 2022, 68 final datasets from published studies are available for download. In 2021, 2,492 users accessed the webpage 6,967 times and downloaded 731 datasets!
While providing these data, the CIBMTR is committed to safeguarding the privacy of participants and protecting confidential and proprietary data. Upon accessing the datasets page on the CIBMTR public website, the viewer is notified that the dataset was collected by the CIBMTR, and the CIBMTR's supporters are listed. The webpage also clearly notes the terms and conditions of dataset usage.
Individuals who analyzed the datasets have found them very useful. Below, Amy Keating, MD, from The University of Colorado shares her experience with a dataset.
"For children with AML, one key and unresolved question is the number of cycles of chemotherapy that should be delivered prior to transplantation. The benefits of extra chemotherapy may be to deepen the remission, but the risks are that additional (and potentially unnecessary) chemotherapy may cause toxicity that could either preclude or worsen transplant outcomes. This issue is now further complicated by MRD testing, which likely now also clinically impacts the decision to move to transplantation. For children with AML, typically either two or three cycles are planned, but this number is largely empiric.
To shed light on this issue, we have turned to the newly established CIBMTR Datasets for Secondary Analysis and have used Dr. Muna Qayed's data set from the publication 'A validated pediatric disease risk index for allogeneic hematopoietic cell transplantation' (Blood, 2020). This dataset contains key data (time from diagnosis to transplant and pre-transplant MRD status), which will allow us to address the hypothesis that regardless of MRD status a third cycle of chemotherapy does not improve transplant outcomes." – Amy Keating, MD, Associate Professor of Pediatrics, The University of Colorado, School of Medicine; Medical Director, Clinical Blood and Marrow Transplant and Cellular Therapeutics Program
NEW datasets are now available online.
Return to TOP
Acute Leukemia Working Committee
Committee Leadership
Co-Chairs:
- Mark Litzow, MD, Mayo Clinic Rochester, Rochester, MN
- Partow Kebriaei, MD, M.D. Anderson Cancer Center, Houston, TX
- Christopher Hourigan, MD, D Phil, National Heart, Lung, and Blood Institute – NIH, Bethesda, MD
Scientific Director:
Statistical Director:
Statistician:
Acute leukemia remains the most common indication for allogeneic HCT. This is partially due to increasing numbers of older adults receiving HCT along with improved access due to expanded donor availability. However, post-HCT relapse remains a major barrier. The increasing use of molecular profiling is helping to inform therapy and risk prognostication. The CIBMTR has supported this effort by extending data collection to include questions regarding cytogenetics and molecular markers to allow for more informative and relevant transplant registry studies. The upcoming 2022 Pre-TED summer release will expand the collection of MRD test details, including flow cytometry, cytogenetic, and fluorescence in situ hybridization (FISH), next-generation sequencing, and polymerase chain reaction. We will also start collecting data on mutations and molecular abnormalities associated with Ph-like ALL. The emergence of post-HCT therapies to prevent relapse has added another setting for data collection. We expect many debates and analyses proposed on these and other topics in the Acute Leukemia Working Committee. HCT indications, approaches, and comparison with other therapies are all valid topics for study.
The success of the Acute Leukemia Working Committee predominantly derives from a dedicated team with collaborative input. Committee leadership works closely with committee members, data managers, and transplant groups in both the US and abroad. Leadership develops and promotes the scientific agenda (established with input from committee members); determines priorities in the selection of high-impact studies; and ensures timely progress in protocol development, statistical analyses, manuscript development, and publications. The committee strives to improve quality and efficiency and is guided by the three principles established by the CIBMTR Advisory Committee: Publish peer-reviewed papers of high scientific impact, complete studies within a reasonable time period, and ensure inclusiveness and fairness within the study process.
The Acute Leukemia Working Committee's recent academic activity includes a presentation at the 2021 ASH Annual Meeting (Boyiadzis et al, "Prompt CR plus consolidation therapy yields improve survival after allogeneic transplantation for AML patients receiving myeloablative, but not RIC: A CIBMTR analysis") and six manuscripts published during 2021 in Leukemia, Blood Advances, Bone Marrow Transplant, and Transplantation and Cellular Therapy. Topics investigated included risk assessment using an adapted LeukemiaNet stratification1 or based on the depth of complete remission2 or the type of KMT2A-mutation in AML3. Methany et al. highlighted the challenge of relapse in persons undergoing HCT for therapy-related MDS or AML4. Widuewilt et al. examined the impact of donor source on HCT outcomes for ALL5 and, in a separate publication, compared outcomes of older adolescents / young adults who received pediatric-style chemotherapy (CALBG 10403 trial) or myeloablative HCT for ALL in first complete remission6. In addition, the committee received nearly 50 proposals in anticipation of the 2022 Tandem Meetings of ASTCT and CIBMTR. These numbers reflect a rapidly evolving field and a high level of interest in the rich data on acute leukemia within the CIBMTR Research Database. As a committee, we are eager to review and discuss new proposals from centers worldwide.
Number of Cases in the CIBMTR Research Database
Scroll table to view all
References
- Jimenez Jimenez AM, De Lima M, Komanduri KV, et al. An adapted European LeukemiaNet genetic risk stratification for acute myeloid leukemia patients undergoing allogeneic hematopoietic cell transplant. A CIBMTR analysis. Bone Marrow Transplantation. 2021 Dec 1; 56(12):3068-3077. doi: 10.1038/s41409-021-01450-3. Epub 2021 Sep 28.
- Percival ME, Wang HL, Zhang MJ, et al. Impact of depth of clinical response on outcomes of acute myeloid leukemia patients in first complete remission who undergo allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation. 2021 Sep 1; 56(9):2108-2117. doi: 10.1038/s41409-021-01261-6. Epub 2021 Apr 16.
- Menghrajani K, Gomez-Arteaga A, Madero-Marroquin R, et al. Risk classification at diagnosis predicts post-HCT outcomes in intermediate-, adverse-risk, and KMT2A-rearranged AML. Blood Advances. 2021 Sep 22; bloodadvances.2021004881. doi: 10.1182/bloodadvances.2021004881. [Epub ahead of print].
- Metheny L, Callander NS, Hall AC, et al. Allogeneic transplantation to treat therapy-related myelodysplastic syndrome and acute myelogenous leukemia in adults. Transplantation and Cellular Therapy. 2021 Nov 1; 27(11):923.e1-923.e12. doi:10.1016/j.jtct.2021.08.010. Epub 2021 Aug 21.
- Wieduwilt MJ, Yin J, Wetzler M, et al. Dasatinib and dexamethasone followed by hematopoietic cell transplantation for adults with Ph-positive ALL. Blood Advances. 2021 Nov 23;5(22):4691-4700. doi: 10.1182/bloodadvances.2021004813. PMC8759134.
- Wieduwilt MJ, Stock W, Advani A, et al. Superior survival with pediatric-style chemotherapy compared to myeloablative allogeneic hematopoietic cell transplantation in older adolescents and young adults with Ph-negative acute lymphoblastic leukemia in first complete remission: analysis from CALGB 10403 and the CIBMTR. Leukemia, 2021Jul 1; 35(7):2076-2085. doi: 10.1038/s41375-021-01213-5. Epub 2021 Mar 30. PMC8257494.
View planned, in-progress, and completed studies and publications on the Acute Leukemia Working Committee webpage.
Return to TOP
2022 Tandem Meetings
By Tia Houseman

Tandem Meetings | Transplantation and Cellular Therapy Meetings of ASTCT™ and CIBMTR® (Tandem Meetings) are the combined annual meetings of the American Society for Transplantation and Cellular Therapy (ASTCT) and the Center for International Blood & Marrow Transplant Research (CIBMTR). Administrators, clinicians, data manager/clinical research professionals, fellows-in-training, investigators, laboratory technicians, MD/PhDs, nurses, nurse practitioners, pharmacists, physician assistants, and other allied health professional attendees benefit from a full scientific program that addresses the most timely issues in hematopoietic cell transplantation and cellular therapy.
After careful consideration, ASTCT and CIBMTR have decided to postpone the 2022 Tandem Meetings scheduled for February 2-6, 2022, until April 23-26, 2022. We look forward to seeing you in Salt Lake City, Utah. The full postponement announcement sent on January 12 is included below. To get the announcement out to the Tandem Meetings community as fast as possible, we do not have all details ironed out and appreciate your patience as we work through these details during the upcoming weeks. Please continue to check the FAQ page located on the Tandem Meetings site.
Condensed Footprint
The 2022 Tandem Meetings in April will have a slightly different look and feel in comparison to previous years. We are excited to provide the opportunity to meet in person and for those unable to travel, we will offer more enhanced virtual options. All registered attendees will have access to view recordings at their leisure post-meeting.
In addition, there will be some meetings that will take place before the April dates; we will notify you of those meetings and how to access them within the online agenda as this information becomes available.
Postponement Announcement of the 2022 Tandem Meetings
After careful consideration, ASTCT™ and CIBMTR® have decided to postpone the 2022 Tandem Meetings | Transplantation and Cellular Therapy Meetings of ASTCT™ and CIBMTR® originally scheduled for February 2-6, 2022 to April 23-26, 2022.
While we are disappointed to come to this decision, we concluded that it is the best option to enable in-person attendance for as many of you as possible, and at the same time, ensure comprehensive virtual opportunities if you are unable to join us in Salt Lake City.
ASTCT and CIBMTR place the highest priority on health and safety, and we are confident that this decision is in the best interest of our attendees, supporters, speakers, and staff, as well as the patients you serve. The decision was not made lightly, as we know moving the meetings to a later date will have an impact on your future plans. We appreciate your understanding as we continue to navigate this evolving situation.
Registration: Current registrations will be transferred. Registration will remain open for those not already registered. For those unable to travel, there will be a virtual option, and all registered attendees will have access to view session recordings of all educational tracks after the conclusion of the meeting.
Hotel: Housing reservations for February will be canceled for you without penalty fees and you will receive a confirmation email. Additional details will be sent in the near future on how to book housing for the April dates.
Shipping: If you have already shipped items to the advanced warehouse and it has been received, it will be held until April. Anything sent that has not already been received will be refused and returned.
Flights: If you have already booked your travel for Salt Lake City, we urge you to reschedule your flights and travel plans as soon as possible.
ASTCT and CIBMTR are in the process of moving the scientific program to the April dates under a condensed footprint. Some sessions may take place online in the months leading up to the meetings. You can reference the 2022 Tandem Meetings FAQ for additional information. We will share more information as it becomes available.
Both organizations remain committed to providing a safe, healthy environment, so masks and vaccination are still required to attend the 2022 Tandem Meetings this April. We will continue to collaborate with CLEAR Health Pass to offer quick, easy, and secure health screenings on-site. You can read more about our health and safety policies on the 2022 Tandem Meetings website.
Thank you for your understanding and patience as we continue to navigate the impacts of COVID-19. Please contact TandemMeetings@mcw.edu with questions.
We look forward to seeing you in Salt Lake City, Utah, April 23-26, 2022!
Thank you,
2022 Tandem Meetings co-Chairs
Katharina Fleischhauer, MD, University Hospital Essen
Margaret MacMillan, MD, MSc, FRCPC, University of Minnesota
Support Opportunities and Additional Information
Please direct any questions regarding support opportunities at the 2022 Tandem Meetings of ASTCT & CIBMTR to the Tandem Meetings Conference Office: TandemMeetings@mcw.edu.
We look forward to seeing you!
Return to TOP
BMT CTN Celebrates 20 Years
By Amy Foley, MA

The BMT CTN turned 20 years old in September! The initial goal was to establish a network to accelerate research in HCT by comparing novel therapies to existing ones. Twenty years, more than 15,500 patients enrolled on 59 trials, and 137 publications later…I think we can all agree it's been a success!
Words used by the Steering Committee when asked the best thing about the BMT CTN
The current grant cycle ends in 2024, and right now we are unsure what the long-term future holds. But to finish this current cycle strong, the BMT CTN is committed to completing 15 launched studies (just activating, accruing, or in follow-up). Areas of focus will also be to enhance Diversity, Equity, and Inclusion initiatives; promote Early Stage Investigator involvement; and implement Patient and Caregiver Task Force recommendations. We are hopeful this enhanced foundation, with input from more voices throughout the entire clinical trial process, along with the Network's established track record, will make it clear the BMT CTN should be renewed for another grant cycle.
The BMT CTN is grateful to the NHLBI and NCI for their continued support, especially from our Project Officers, who champion our work. The Network's accomplishments are due first and foremost to the patients who have participated in BMT CTN studies. And to the multitudes of you who have helped grow the BMT CTN into what it is today, thank you. Here's to another 20 years!
About the BMT CTN
The CIBMTR (at both MCW and NMDP) shares administration of the BMT CTN Data and Coordinating Center with other departments of NMDP/Be The Match and with The Emmes Company. Together, these three organizations support all BMT CTN activities.
To get up-to-date information about BMT CTN studies, meetings, and news:
Like us on Facebook: www.facebook.com/bmtctn
Follow us on Twitter: @BMTCTN
Return to TOP
Leveraging Bioinformatics Research to Improve Cell Source Selection
By Ray Sajulga
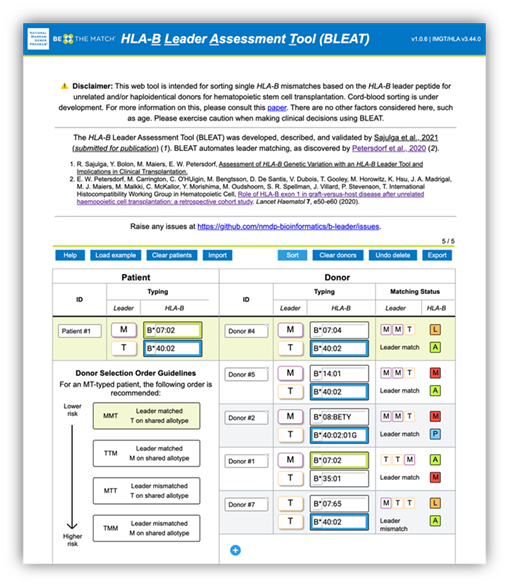
Figure 1. BLEAT provides a rank order for candidate stem cell sources ("donors") to optimize therapy selection and patient outcomes.
We cannot live without technology. From the device you’re presumably using to read this sentence to our ubiquitous online infrastructures, of which we’ve become so dependent (for communicating, shopping, enjoying entertainment, etc.), there is much to benefit from technological innovation. It has certainly helped in our struggle against the COVID-19 pandemic. Moreover, it has improved our vital work here at the CIBMTR to save lives. A few months before the start of the pandemic, when I joined the CIBMTR and the Bioinformatics Research department, I started two projects: HLA-B leader and HLA-DPB1 TCE (T-cell epitope) prediction. These involved cleaning and preparing NMDP/Be The Match datasets, constructing algorithms to transform HLA data, and developing user interfaces to equip transplant centers with novel HLA methodology. In other words, these projects leveraged bioinformatics research (i.e., the application of computation and technology to biology) to improve cell source selection.
Leading the way, the HLA-B leader project revolved around a dimorphic genetic marker on the leader peptide on HLA-B, rs1050458C/T, whose importance was discovered by Petersdorf et al. 20191. This single nucleotide polymorphism encodes either methionine (M) or threonine (T) at P2 (position 2) on the leader peptide, which can influence alloreactive responses. For single HLA-B mismatches (i.e., one matched allele and one mismatched allele), having the same leader P2 allele on the mismatched alleles between recipient and donor, such as HLA-B*07:01 (M) and HLA-B*14:02 (M), can lower the risk of acute GVHD. We can further lower acute GVHD risk in patients who have M and T leader P2 alleles, where the matched leader P2 alleles are T alleles rather than M alleles1. If you think these rules are complex, think about the many transplant specialists who had to both assign P2 alleles and implement these matching rules by hand. As you can imagine, this can be tedious. As a solution, in collaboration with Effie Petersdorf, MD, co-authors, and colleagues, we developed a tool called BLEAT: HLA-B Leader Assessment Tool. BLEAT automates the assignment, matching, and sorting of HLA-B mismatches to optimize cell source selection. More details are in our manuscript, which also details the application of BLEAT to 9.4 million donors with HLA-B typing, 1.1 million donors with HLA-B leader sequences, and 1,259 donor-recipient transplants2:
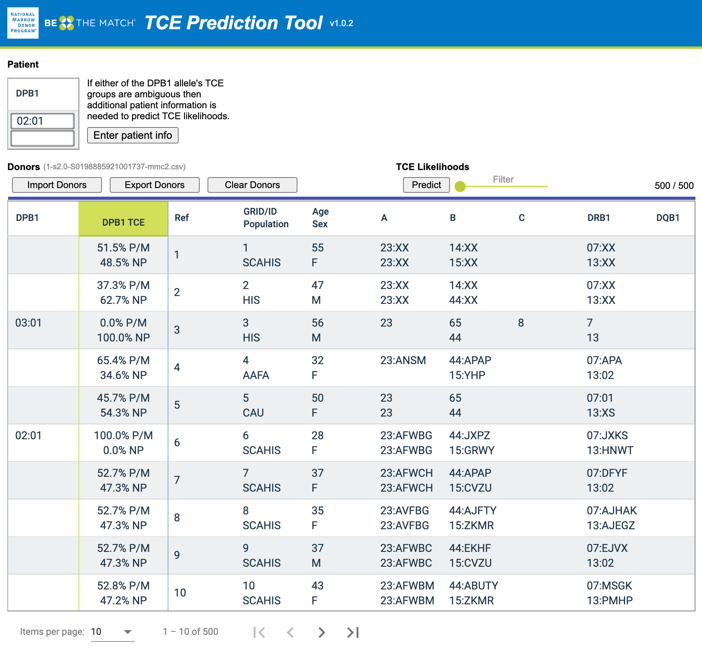
Figure 2. A TCE (T-Cell Epitope) Prediction Tool screenshot where users provide patient HLA typing (HLA-DPB1*02:01+HLA-DPB1*02:01) and an exported donor list (from the NMDP's MatchSource® matching platform) with HLA typing and population codes as inputs
Secondly, HLA-DPB1 TCE Prediction focused on improving the selection of cell sources, e.g., unrelated donors, based on HLA-DPB1 typing (and TCE groups), which is only available in one out of five donors3. My co-authors and I accomplished this by extending the imputation of existing six-locus HLA typing4 (HLA-A~C~B~DRB3/4/5~DRB1~DQB1) to seven-locus typing (HLA- A~C~B~DRB3/4/5~DRB1~DQB1~DPB1), aggregating the imputed HLA-DPB1 alleles to TCE groups, and calculating the TCE permissive mismatch likelihood. This information is valuable for transplant centers to identify the best donors for confirmatory typing. Furthermore, this can be especially beneficial for populations of color who have less HLA-DPB1 typing available. We developed a TCE Prediction Tool to visualize the permissive likelihood and test it among transplant centers participating in the DOTS (Donor Optimization for Transplant Success) program with positive results. Further details on this project are in our manuscript3:
Both projects are published and accessible to numerous transplant centers worldwide, with more than 380 users across 10 countries. Additionally, these automated models have paved the way for their inclusion into the MatchSourceⓇ matching platform. With newer innovations in HLA matching, like HLA-DPB1 expression, there are more opportunities for leveraging technology to improve matching. There are also possibilities to gather these models into a central hub to synergize the newest innovations in HLA matching to improve patient outcomes. As demonstrated here, through technology, we can save more lives.
References
- Petersdorf EW, Carrington M, O'HUigin C, et al. Role of HLA-B exon 1 in graft-versus-host disease after unrelated haemopoietic cell transplantation: A retrospective cohort study. Lancet Haematology. 2020 Jan 1; 7(1):e50-e60. doi: 10.1016/S2352-3026(19)30208-X. Epub 2019 Oct 25. PMC6948919.
- Sajulga R, Bolon YT, Maiers M, Petersdorf EW. Assessment of HLA-B genetic variation with an HLA-B Leader Tool and implications in clinical transplantation. Blood Advances. 2022 Jan 11; 6(1):270-280.
- doi: 10.1182/bloodadvances.2021004561. PMC8753210.
- Sajulga R, Madbouly A, Fingerson S, et al. Predicting HLA-DPB1 permissive probabilities through a DPB1 prediction service towards the optimization of HCT donor selection. Human Immunology. 2021 Dec 1; 82(12):903-911.
- doi: 10.1016/j.humimm.2021.06.010. Epub 2021 Aug 3.
- Gragert L, Madbouly A, Freeman J, Maiers M. Six-locus high resolution HLA haplotype frequencies derived from mixed-resolution DNA typing for the entire US donor registry. Human Immunology. 2013 Oct 1; 74(10):1313-1320. doi: 10.1016/j.humimm.2013.06.025. Epub 2013 Jun 24.
Return to TOP
SCTOD: Center-Specific Survival Analysis and Center Outcomes Forum
By Carol Doleysh
The SCTOD is part of the US Health Resources and Services Administration (HRSA)-funded C.W. Bill Young Cell Transplantation Program that collects data on all allogeneic HCTs performed in the US and on transplants done elsewhere using cellular products that originated in the US.
Outcomes reporting in allogeneic HCT is necessary to provide the information requested by patients, insurers, and government agencies as well as to comply with current laws. The SCTOD contract requires the CIBMTR to conduct an annual analysis of one-year survival rates at each transplant center in the US. The report generated by the CIBMTR is meant to be used as a quality improvement tool for transplant centers. The data are also made available to the public at http://bethematch.org/tcdirectory/search. For inclusion in the analysis, transplant centers must have at least one year of follow-up on more than 90% of related and unrelated HCT recipients within the reporting period. A description of the methodology used in generating this report can be found on the CIBMTR's website. Following recommendations from the 2020 Center Outcomes Forum, recipients of HCT in 2019 who developed COVID-19 infection during their first year of follow-up were censored in the model for the 2021 analysis.
The 2021 Center-Specific Survival Report, which includes first allogeneic HCT performed between 2017 and 2019 in the US, was distributed in mid-December to center directors, payers, and FACT. The data were also updated on the Be The Match® website. Additional tools accessible to transplant centers for quality improvement include Center Performance Analytics and the Survival Calculator. Access to these tools is through the secure CIBMTR Portal, where the Center-Specific Survival Report is also available to transplant centers. Any questions regarding CIBMTR Portal account credentials should be submitted via CIBMTR Center Support (https://nmdp.service-now.com/csm), by selecting CIBMTR Center Maintenance then CIBMTR Portal Help.
The 2022 Center-Specific Survival Report will include patients who received HCT between 2018 and 2020. Inclusion of HCT initiated in 2020, during the COVID-19 pandemic, means these recipients may have been affected differently than those transplanted in 2019 who may have developed COVID-19 during 2020. On November 12, 2021, the CIBMTR held a Center Outcomes Forum to discuss risk adjustment for the impact of the COVID-19 pandemic in the 2022 Center-Specific Survival Analysis. Participants included representatives of the HCT community, including transplant physicians and center directors, the ASTCT and its Quality Outcomes Committee, FACT, governmental funding agencies, patients, private payors, and statisticians. The CIBMTR distributed a summary of the meeting to US Medical Directors and posted it on the CIBMTR website.
Based on recommendations from the 2021 Center Outcomes Forum, the CIBMTR is planning to complete 3 exploratory analyses to examine the impact of the pandemic on HCT practice changes, examine the impact of geographic COVID-19 incidence and time period on post-HCT survival, and examine the impact of COVID-19-associated practice changes on post-HCT survival. (Details of all exploratory analyses are included in the posted meeting slides and described in the meeting summary.) Based on the results of the exploratory analyses, a final approach to the 2022 Center-Specific Survival Analysis will be determined. US centers are being asked to provide necessary data about changes in HCT procedures, COVID infection in patients, and follow-up on an accelerated schedule this year to make these additional analyses possible.
Return to TOP
Our Supporters
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; U24CA233032 from the NCI; OT3HL147741 and U01HL128568 from the NHLBI; HHSH250201700005C, HHSH250201700006C, and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2832 and N00014-21-1-2954 from the Office of Naval Research.
Additional federal support is provided by P01CA111412, R01CA100019, R01CA152108, R01CA218285, R01CA231141, R01CA231838, R01AI128775, R01AI158861, R01HL130388, R01HL155741, R01HL131731, U01AI069197, U01AI126612, UG1HL06924.
Support is also provided by Be the Match Foundation; Boston Children's Hospital; Dana Farber; St. Baldrick's Foundation; PBMTF; Stanford University; Medical College of Wisconsin; National Marrow Donor Program; and from the following commercial entities: AbbVie; Accenture; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allovir, Inc.; Amgen, Inc.; Astellas Pharma US; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Fate Therapeutics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon; Karius; Karyopharm Therapeutics; Kiadis Pharma; Kite Pharma Inc; Kite, a Gilead Company; Kyowa Kirin International plc; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Medac GmbH; Medexus; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OncoImmune, Inc.; Oncopeptides, Inc.; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc; Pfizer, Inc.; Pharmacyclics, LLC; Priothera; Sanofi Genzyme; Seagen, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Tscan; Vertex; Vor Biopharma; Xenikos BV.
Return to TOP